Introduction:Zeta Potential
3.What is the "electro-osmosis effect" that is important for measuring the zeta potential?
The isoelectric point of quartz, which is the material of the cell, is pH 2 to 3, and the cell surface is negatively charged at pH higher than that. Therefore, positively charged ions and particles gather near the cell wall and when an electric field is applied, these ions cause a flow to the negative electrode side near the wall surface. As a result, the reverse flow to the positive electrode is generated at the center of cell in order to compensate for the flow.
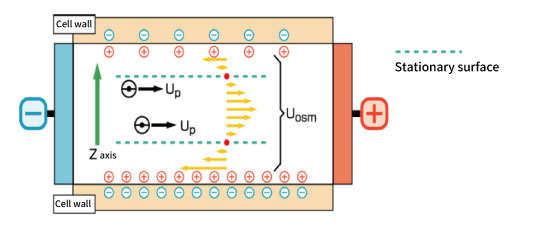
Uobs(Z) = Up + Uosm(Z)
This series of flows is called an electroosmotic flow, and when zeta potential measurement is performed, the generation of this electroosmotic flow is unavoidable. As a result, the observed apparent electrophoresis includes the electroosmotic flow.
Uobs(Z) = AU0(z/b)2+ΔU0(z/b)+(1-A)U0+Up ・・・(4)
A = 1/ [ (2/3) - (0.420166/k) ]
k = a/b : Ratio of the length a and b of the cell cross section(a>b)
U0 : Average flow velocity of the solvent at the top and bottom of the cell
ΔU0 : Difference in flow velocity of the solvent at the top and bottom of the cell
However, the surface potential of cell changes depending on the pH of a solution, adsorption of samples and additives, sedimentation of particles, cell cleaning method, etc., and often generates a vertically asymmetric electroosmotic flow. When it becomes asymmetric, the true mobility cannot be obtained by the measurement on a stationary surface, which is generally obtained from the cell shape.
ELSZ series observe the apparent electrical mobility of particles affected by the electroosmotic flow at several points of the cell, and recalculate the stationary surface from equation (4). Therefore, the true mobility can be obtained even in a system where the electroosmotic flow is asymmetric.
In addition, this measurement method can be used to determine the charge state of the surface of cell. Using a flat surface cell, attach the flat plate sample to one side of the cell and observe the electroosmotic flow that reflects the surface potential of the flat plate sample. By analyzing this, the zeta potential of the flat plate sample can be obtained.
(Revised in September, 2022)
Related Products
| Zeta-potential & Particle size Analyzer ELSZneo | |
| Zeta-potential & Particle size Analyzer ELSZ-2000 series |


 Close
Close



