Zeta potential of the polymer electrolyte laminated film
2.Experiment
1) Experimental sample
The cationic polyelectrolyte polyethyleneimine (manufactured by MP Biomedicals, Mw: 50,000 to 100,000) and the anionic polyelectrolyte polystyrene sodium sulfonate (manufactured by Aldrich, Mw: 75,000) were used. The Micro Slide Glass (manufactured by IWAKI) was used as the flat plate sample. The surface zeta potential was measured using Zeta potential / particle size measurement system ELSZ series manufactured by Otsuka Electronics Co., Ltd.
2) Experimental method
First, the surface zeta potential of slide glass was measured as a blank. After the slide glass was set in a flat surface cell unit, polystyrene latex, which was coated with hydroxypropyl cellulose so that the surface charge is almost zero, dispersed in a 10 mM NaCl solution was injected to the cell as monitor particles. Then they were electrophoresed and the apparent velocity distribution inside the cell was measured by the laser Doppler method. Since the particles can be approximated to zero charge, their velocity distribution represents the electroosmotic flow generated in the liquid in contact with the flat plate sample that has the potential on its surface. The surface zeta potential of the slide glass can be obtained by theoretically analyzing the apparent velocity distribution in the cell using Mori and Okamoto's equation2). Refer to the reference 3) for the details of the measurement method. Fig. 1. shows a photograph of the flat surface cell unit and its side view.
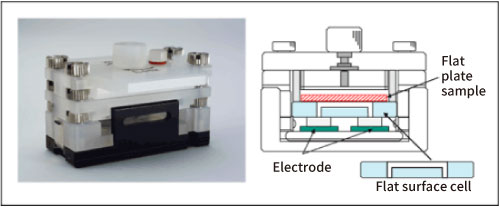
Fig. 1. Flat surface cell unit and its side view
Next, the slide glass was taken out, the surface was thoroughly washed with distilled water, soaked in a polyethylene imine aqueous solution having a concentration of 4 × 10-4 mol/L for an hour, then thoroughly washed with distilled water. The monitor particles were injected to the cell and the surface zeta potential of slide glass was measured in the same manner. Furthermore, the slide glass was taken out again, washed thoroughly with distilled water, then immersed in a sodium polystyrene sulfonate aqueous solution having a concentration of 4 × 10-4 mol/L for an hour, thoroughly washed with distilled water, the monitor particles were injected to the cell and the surface zeta potential of the slide glass was measured in the same manner.
In this way, the operation of alternately immersing in the cationic and anionic polyelectrolytes was repeated four times, and the surface zeta potential of the slide glass was measured each time.
| References | 2) | Hiroyuki Mori, Yoshio Okamoto, Froth flotation, 27 (3) 117-124 (1980) |
| 3) | Shoichi Nakamura, LS Advance, 2 (1) 27-30 (2003) |


 Close
Close

